Abstract
Introduction: Isocitrate dehydrogenase 2 (IDH2) mutations (mIDH2), which typically occur at arginine residues R140 and R172, induce production of the oncometabolite 2-hydroxyglutarate (2-HG), causing aberrant hypermethylation of DNA and histones and blockade of differentiation. In vitro, combining ENA, an mIDH2 inhibitor, plus AZA showed synergistic effects, leading to greater-than-additive reductions in DNA methylation and enhanced differentiation. In a phase 2 trial (NCT02677922), combination treatment (Tx) with ENA + AZA significantly improved morphologic response rate compared with AZA monotherapy in adult patients (pts) with mIDH2 newly diagnosed (ND) AML. We investigated the association between molecular characteristics and clinical outcomes with ENA + AZA and AZA only in that trial.
Methods: Total 2-HG in peripheral blood was assessed by liquid chromatography-mass spectrometry (Covance) at baseline (BL), cycle (C) 1 day (D) 15, C2D1, C2D15, and on D1 of every other cycle from C3 to C19. IDH2 variant allele frequency (VAF) was quantified in DNA from bone marrow mononuclear cells by digital PCR (Sysmex) and co-occurring mutations were identified by targeted next-generation sequencing using a 37-gene myeloid panel (ArcherDx). Gene VAFs (including IDH2) were assessed at BL and on D1 of C2, C3, C5, C11, and C17. Clinical response categories included complete remission (CR), incomplete response (IR; ie, non-CR response), or no response (NR; ie, stable or progressive disease), per IWG 2003 response criteria for AML. Statistical analyses were performed using GraphPad Prism v.8.0.0 and R v.4.1.0.
Results: In all, 101 pts were randomized to ENA + AZA (n = 68) or AZA only (n = 33). At BL, most pts (74%) had an IDH2-R140 mutation. SRSF2, RUNX1, FLT3-ITD, NPM1, and SF3B1 were significantly (P ≤ 0.05) preferentially co-mutated with IDH2-R140 (vs R172), and DNMT3A and ETV6 were preferentially co-mutated with IDH2-R172 (vs R140). BL 2-HG levels and IDH2 VAFs were similar between treatment arms and IDH2 variants, and were not significantly different between clinical response categories (CR/IR/NR).
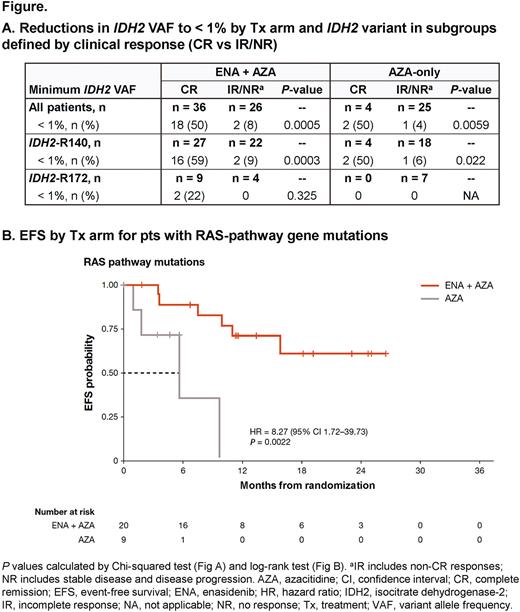
In the ENA + AZA arm, median 2-HG was significantly reduced from BL by C1D15 (n = 60; -91%; P ≤ 0.001) with maximal reduction at C5 (n = 43; -95%; P ≤ 0.001). IDH2 VAF was significantly reduced from BL by C3 (n = 39; -23%; P ≤ 0.01) with maximal reduction at C17 (n = 7; -99%; P ≤ 0.05). In the AZA-only arm, 2-HG was not significantly reduced from BL at any time and IDH2 VAF was significantly reduced only at C11 (n = 7; P < 0.05). Marked IDH2 VAF reductions were observed primarily in complete responders, reaching undetectable levels (ie, VAF < 1%) in 50% (18/36) of pts who achieved CR with ENA + AZA (P = 0.0005 vs IR/NR), and the rate of undetectable IDH2 was higher in the IDH2-R140 subgroup vs R172 (59% vs 22%, respectively). In the AZA-only arm, 2/4 CR pts (both IDH2-R140) had undetectable IDH2 mutations (Figure A).
In both Tx arms, achievement of clinical response was associated with VAF reductions in the combined DNA methylation gene pathway (IDH1/2, DNMT3A, TET2) and receptor tyrosine kinase (RTK) pathway (KIT, CSF3R, FLT3, JAK2), with maximal reductions from BL of ~85% and 100%, respectively, by C11 with ENA + AZA (n = 19 and n = 15; both P ≤ 0.001), and maximal reductions in the AZA-only arm of 50% at C17 (n = 2) for DNA methylation and 100% at C5 (n = 4) for RTK (statistical comparisons not reported due to small numbers). Pts who achieved CR with ENA + AZA also showed a significant VAF reduction in the RAS gene pathway (CBL, PTPN11, N/KRAS), with maximal reduction from BL of 100% by C5 (P ≤ 0.001); the AZA-only arm was not evaluable because very few pts responded. Among all pts with RAS pathway mutations, ENA + AZA significantly prolonged event-free survival vs AZA-only (P = 0.002) (Figure B).
Conclusions: Outcomes in this mIDH2 ND-AML cohort demonstrate robust clinical validation of the synergistic effects of ENA and AZA seen in vitro. BL co-mutational patterns differed between the IDH2-R140 and R172 variants. Rapid reductions in 2-HG and IDH2 VAF were observed during combination Tx with ENA + AZA. Significant differences in undetectable IDH2 VAF were observed between complete and non-complete responders and were more common in pts with IDH2-R140 mutations than with R172. Additional research is needed to further elucidate the benefits of ENA + AZA in pts with RAS pathway mutations, which are associated with resistance to ENA and AZA monotherapies.
Disclosures
Risueño:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. See:Bristol Myers Squibb: Other: Contractor. DiNardo:GenMab: Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; LOXO: Research Funding; Novartis: Honoraria; AbbVie: Consultancy, Research Funding; ImmuneOnc: Honoraria, Research Funding; Takeda: Honoraria; Cleave: Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Honoraria; Servier: Consultancy, Honoraria, Research Funding; Astellas: Honoraria; Foghorn: Honoraria, Research Funding; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria; Gilead: Honoraria; Forma: Research Funding. Döhner:Astellas Pharma Inc.: Consultancy, Honoraria, Research Funding; AstraZeneca: Honoraria; Berlin-Chemie AG: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Novartis AG: Consultancy, Honoraria, Research Funding; Daiichi Sankyo Co, LTD: Consultancy, Honoraria; Gilead Sciences, Inc.: Consultancy, Honoraria; Janssen Pharmaceuticals: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Syndax Pharmaceuticals Inc.: Consultancy, Honoraria; Kronos Bio, Inc.: Research Funding; Pfizer Inc.: Research Funding; Amgen Inc.: Consultancy, Honoraria, Research Funding; Agios Pharmaceuticals: Consultancy, Honoraria, Research Funding; AbbVie Inc.: Consultancy, Honoraria, Research Funding. Stein:Auron Therapeutics: Current equity holder in private company; PinotBio, Bristol Myers Squibb, Jazz Pharmaceuticals, Foghorn Therapeutics, Blueprint Medicines, Gilead Sciences, Janssen Pharmaceuticals: Consultancy; Astellas Pharmaceutical, Agios Pharmaceuticals, and Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo, Celgene Pharmaceuticals, and Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PTC Therapeutics and Syros: Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Research Funding; Bayer: Research Funding; Amgen, AbbVie, Seattle Genetics, and Biotheryx: Consultancy. Fathi:Abbvie/Servier: Consultancy, Other: Clinical Trial Support; Novartis: Consultancy; Amgen: Consultancy; Celgene/BMS: Consultancy, Other: Clinical Trial Support; Forma: Consultancy; Ipsen: Consultancy; Morphosys: Consultancy; Foghorn: Consultancy; Immunogen: Consultancy; Mablytics: Consultancy; Kite: Consultancy; Astellas: Consultancy; Orum: Consultancy; EnClear: Consultancy; PureTech: Consultancy; AbbVie, Agios, Bristol Myers Squibb, Servier, and Takeda: Research Funding; AbbVie, Agios, Amgen, Astellas Pharma, Blueprint Medicines, Bristol Myers Squibb, Daiichi Sankyo, Foghorn Therapeutics, Forty Seven, Inc., Genentech, Ipsen, Kite Pharma, Kura Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; MorphoSys, Novartis, Pfizer, Seattle Genetics, Takeda, Trillium Therapeutics, and Trovagene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Genentech: Consultancy. Vyas:Bristol Myers Squibb: Research Funding; Abbvie: Honoraria; Astellas: Honoraria; Celgene: Honoraria, Research Funding; Daiichi Sankyo: Honoraria; JAZZ: Honoraria; Pfizer: Honoraria. Quek:Jazz Pharmaceuticals: Speakers Bureau; Servier: Research Funding; Bristol Myers Squibb: Research Funding. Prebet:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Gandhi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Hasan:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal